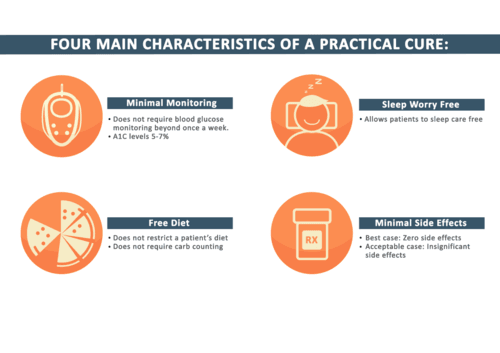
This report reveals that of the 329 type 1 human clinical trials, there are just six with the potential to deliver a Practical Cure. While one project was added to this list since 2011, a greater focus on Practical Cure work will push more projects into human clinical trials.
This report discusses the actions that donors can take to increase funding for Practical Cure research. This area of research receives little attention from the major diabetes non-profits; only three cents of every cure research dollar are applied to Practical Cure research.
This report is the inaugural publication of what will be a yearly update series. It summarizes the findings from many of our reports to create a comprehensive picture of factors that impact cure research and how we as donors can impact progress. The report shows that there is much more that affects cure development than simply research in a laboratory. How is research chosen? How is money raised? How are donor contributions utilized? What scientific milestones have been accomplished? The report addresses all of these topics.
This report analyzes the cure message that diabetes non-profits use to solicit contributions at fundraising events. The JDCA reviewed over 400 fundraising events that the major diabetes non-profits organized in the United States in 2012.
This report provides an introduction to the infrastructure that supports type 1 diabetes research in the United States. With the donors’ interest in mind, we follow the money trail from its source to the laboratory.
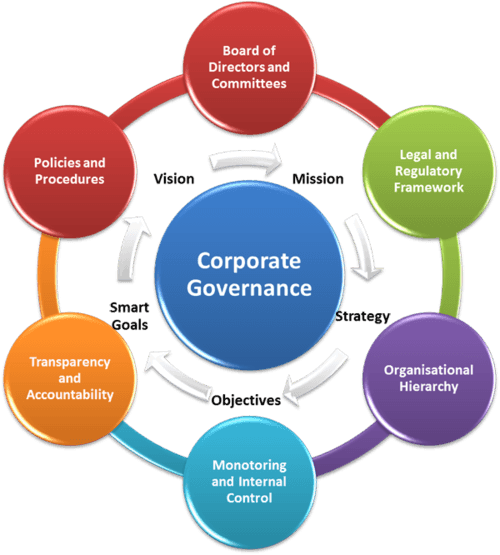
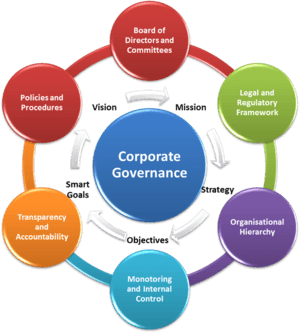
This report, the fifth in our corporate governance series, examines the role of the non-profits’ governing Board and its effectiveness in the pursuit of a type 1 cure.
This is the third report in an ongoing series of analyses on corporate governance among the four non-profits in the
JDCA’s coverage universe. This report rates the non-profits’ proficiency in four key corporate governance areas: financial data, strategic direction, cure progress, and enterprise governance.
Executive compensation is a critical element of corporate governance. This report, the fourth in our corporate governance series, examines the degree to which financial incentives are a factor in the development of a cure for type 1.
This report examines the organizations’ approach to funding type 1 cure research and proposes an alternative strategy that could accelerate the development of a type 1 cure.
This report slightly longer term view and analyze the trend in funding for type 1 cure research in comparison to the organizations’ funding of other research activities over the past four years.
In this report we analyze the primary messages the major diabetes charities use to solicit donations and examine the relationship between those messages and the non-cure activities that donations fund.
We believe that a lack of transparency exists at not-for-profit (NFP) foundations and that the four diabetes NFPs that we actively follow would benefit from providing reports that follow SEC standards.
Survey data reveals that myths surrounding a cure are widespread and contribute to donor disillusionment and frustration.
The JDCA feels that existing and potential NFP stakeholders would be better served by improved communication frequency, timeliness, and disclosure requirements that more closely mirror the SEC guidelines used by Public Companies.
This report updates and analyzes the charities’ spending in the major expense categories based on their most recent financial statements.
The JDCA believes there are misconceptions relating to prevention research and its relationship to the development of a cure. This report addresses those misconceptions and details why pursuit of prevention research could be detrimental to efforts to cure individuals living with type 1.
This report delineates the reasons why the pursuit of type 1 treatment objectives is best accomplished by commercial enterprises rather than the non-profits.
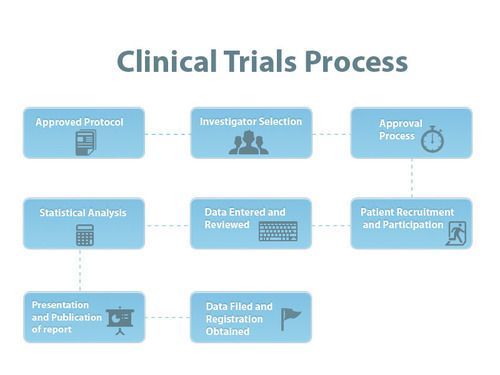
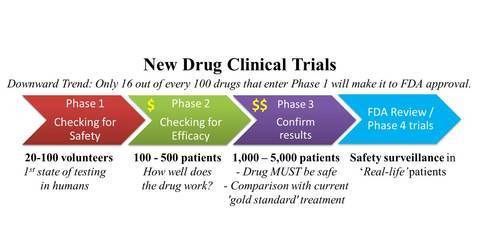
This is the last in a series of three reports that examines the type 1 diabetes (type 1) clinical trial landscape. In this report, we broadly analyze the type 1 trial universe, the focus of research attention and the implications for the development of a type 1 Practical Cure.