This report is the first in a series of updates on developments in Practical Cure research platforms and projects. It outlines the four research pathways that we believe have the potential to deliver a Practical Cure within the next 15 years. In subsequent weeks we will publish the latest lists of projects by pathway that are either currently in human trials or will be in human trials within the next 12 months.
Outcomes of a Practical Cure
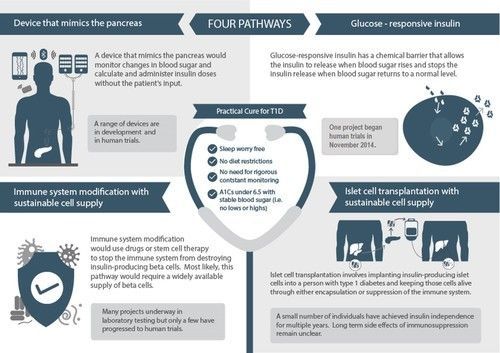
Each of the four research pathways takes a different approach to delivering the same set of outcomes. Together these outcomes constitute a Practical Cure: sleep worry-free, no dietary restrictions, no need for constant monitoring, and A1C's under 6.5 with stable blood sugars.
A Practical Cure may not eliminate type 1 diabetes altogether, but it can be achieved in the next 15 years—in time to transform the lives of people who are currently living with the disease. The JDCA focuses on research in human clinical trials because it stands the best chance of reaching this time goal.
THE FOUR PATHWAYS
1) A Device that Mimics the Pancreas, often referred to as an artificial pancreas, is under development at several commercial and academic centers. To be a Practical Cure, a device that mimics the pancreas would require an exceptionally reliable closed-loop system that adapts to each individual.
2) Glucose-Responsive Insulin, aka “smart insulin,” is chemically activated in response to changes in blood glucose. Once injected, smart insulin would remain inactive until blood glucose rises above normal levels. At that point, the chemical component would activate the insulin. Once blood glucose returns to normal, the insulin action would quickly cease, avoiding low blood sugar. To qualify as a Practical Cure, smart insulin would have to last long enough to not require multiple daily injections.
3) Immune System Modification would stop the body’s immune system from attacking the insulin-producing beta cells, either through drugs or stem cell therapy. There is some evidence that established type 1 diabetics have residual beta cells left that could multiply and produce a sufficient amount of insulin, but if this is not the case, blocking the autoimmune attack would need to be combined with islet cell transplantation.
4) Islet Cell Transplantation involves implanting insulin-producing islet cells into a person with type 1 diabetes. It has three major components:
- Cell protection: The islet cells must be protected from the immune attack after they have been implanted in the body. Various encapsulation approaches have been tested in humans with no breakthrough to date. Immune-suppressing drugs are another alternative, but side effects would have to be reduced to qualify as part of a Practical Cure.
- Cell supply: The only proven source of Islet cells is cadavers, which have very limited availability. Research into deriving a sustainable cell supply from human stem cells has seen recent advances but not yet entered human clinical trials.
- Site selection: Islet cells require large supplies of oxygen and nutrients in order to survive. The current protocol is to transplant islet cells in the liver, where most of them die. Other sites, including the stomach lining and the area under the skin, are being tested as alternatives.
