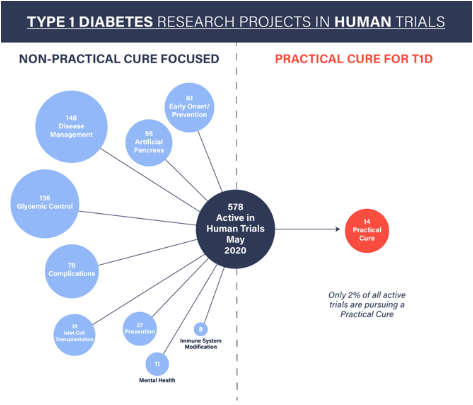
This is an overview of all of the active T1D human trials under the auspices of the U.S. FDA. Multiple human trials are required before any medical treatment, drug, or device can be brought to the U.S. market. No treatment will have a chance to reach this market until it has been tested for safety and efficacy in a human trial registered at clinicaltrials.gov.
The key takeaway from this report is that scientific interest in T1D seems to be on the rise, but only a tiny minority of that research has the potential to cure people who are living with the disease today. In the eight years that the JDCA has conducted this analysis, the total number of T1D human trials has increased substantially – from 329 trials in 2012 to 579 trials in 2020. But in that time, Practical Cure research has never taken up more than a tiny share of researchers' attention.
The Road to FDA Approval
The successive stages through which any research must pass before hitting the market are basic research, translational research, pre-clinical testing on animals, clinical testing on humans, then FDA pre-market approval. On average, this process takes about 15 years.
Human clinical trials include three increasingly stringent levels of testing: Phase I, Phase II, and Phase III. Phase I focuses primarily on the safety of the treatment that is being studied. Phase II and Phase III assess the treatment’s efficacy. Once a treatment has successfully completed Phase III testing, the FDA reviews the research results to determine whether or not to give it approval to go to market.
Current T1D Human Trial Research
As of May 2020, there are 578 active type 1 diabetes projects underway in FDA-sanctioned human trials (including 13 trials that have been suspended due to COVID-19). The vast majority of these trials have nothing to do with curing T1D in the near or short term. Furthermore, only 2% of all T1D human trials have a chance to deliver a Practical Cure – a solution that would free people living with T1D today from the daily burden of the disease.
The current list of active T1D trials breaks down into the following categories:
- 148 disease management trials that examine exercise, diet, technology, or community intervention strategies
- 136 glycemic control trials that test a biological or pharmacological intervention aimed at improving a T1D patient’s ability to manage their blood sugar levels
- 78 complications trials that address retinopathy, stroke, heart failure or other potential health problems that could arise from T1D
- 61 early-onset trials that study or alter the progression of T1D in patients less than two years from their initial diagnosis
- 56 artificial pancreas trials that test systems combining continuous blood glucose monitoring with drug delivery devices
- 39 islet cell transplantation trials that test the surgical transplantation of pancreatic islet cells from a cadaver into a person living with high-risk T1D
- 27 prevention trials that target people at high genetic risk to develop T1D in order to delay or prevent the onset of the disease
- 14 Practical Cure trials that have the potential to completely eliminate the burden of living with T1D within the next 15 years
- 11 mental health trials that aim to improve mental health outcomes for patients with T1D
- 8 immune system modification trials that aim to block, re-train, or somehow modify the immune system of a patient with T1D
Conclusion
In order for a Practical Cure for T1D to become widely available in the U.S., it must first be proven safe and effective in a series of human trials registered with the FDA. The process is time-consuming and many projects will fail to reach the goals set by researchers and regulators. That's why it is crucial that researchers today are incentivized to bring Practical Cure projects into human trials. An immediate increase in Practical Cure-related T1D human trials is the only way to deliver such a solution for people living with the disease today.
