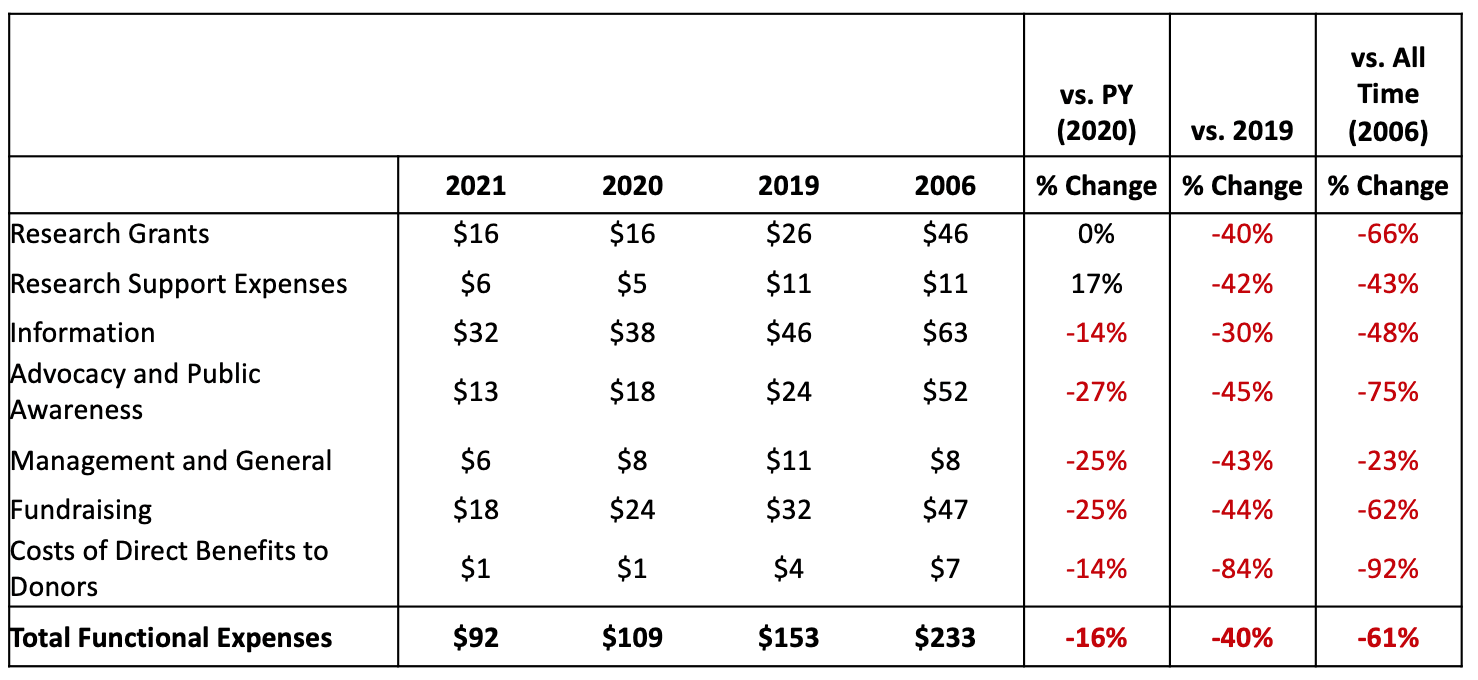Teplizumab, which will go to market by the brand name Tzield, is the first ever disease-modifying therapy for T1D approved by the FDA
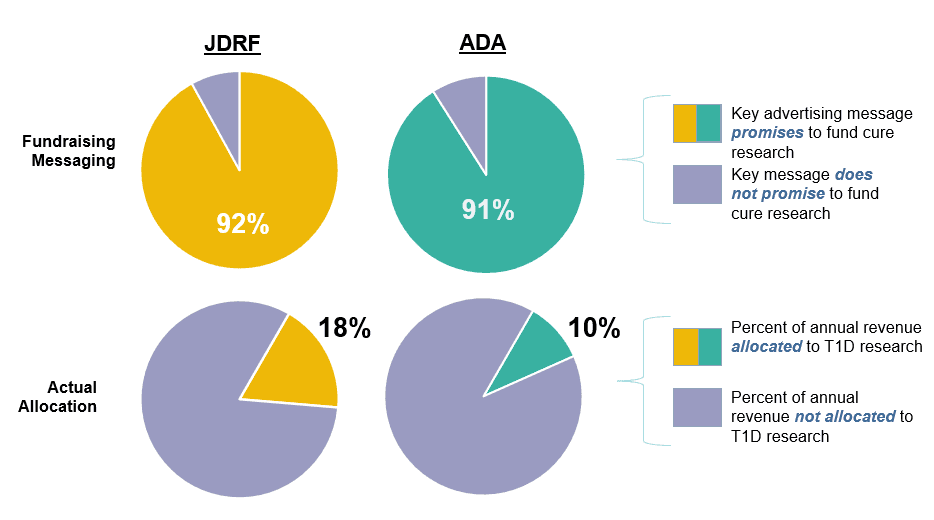
This is the 11th annual report of advertising messaging used by JDRF and the ADA to promote national fundraising events.
This is the 11th annual report analyzing the research grant funding by the Juvenile Diabetes Research Foundation (JDRF).
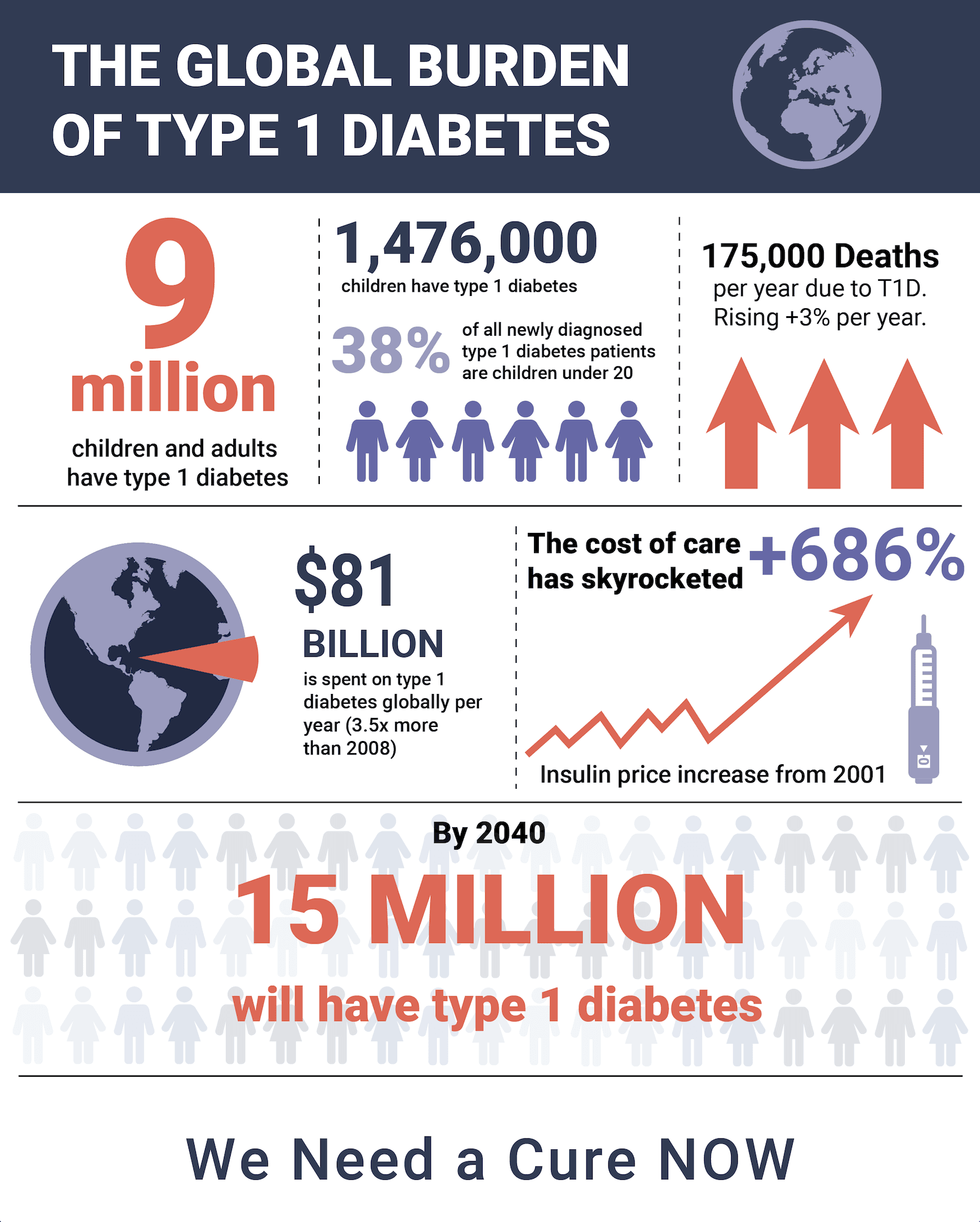
NIH Spends $1.1 Billion on Diabetes Research -- but Zero for a T1D Practical Cure
The individuals who serve as Directors of the JDRF and ADA are the ones who ultimately decide how to allocate the $300+ million donated to T1D research every year. Their decisions and leadership either accelerate or decelerate speed to a cure.
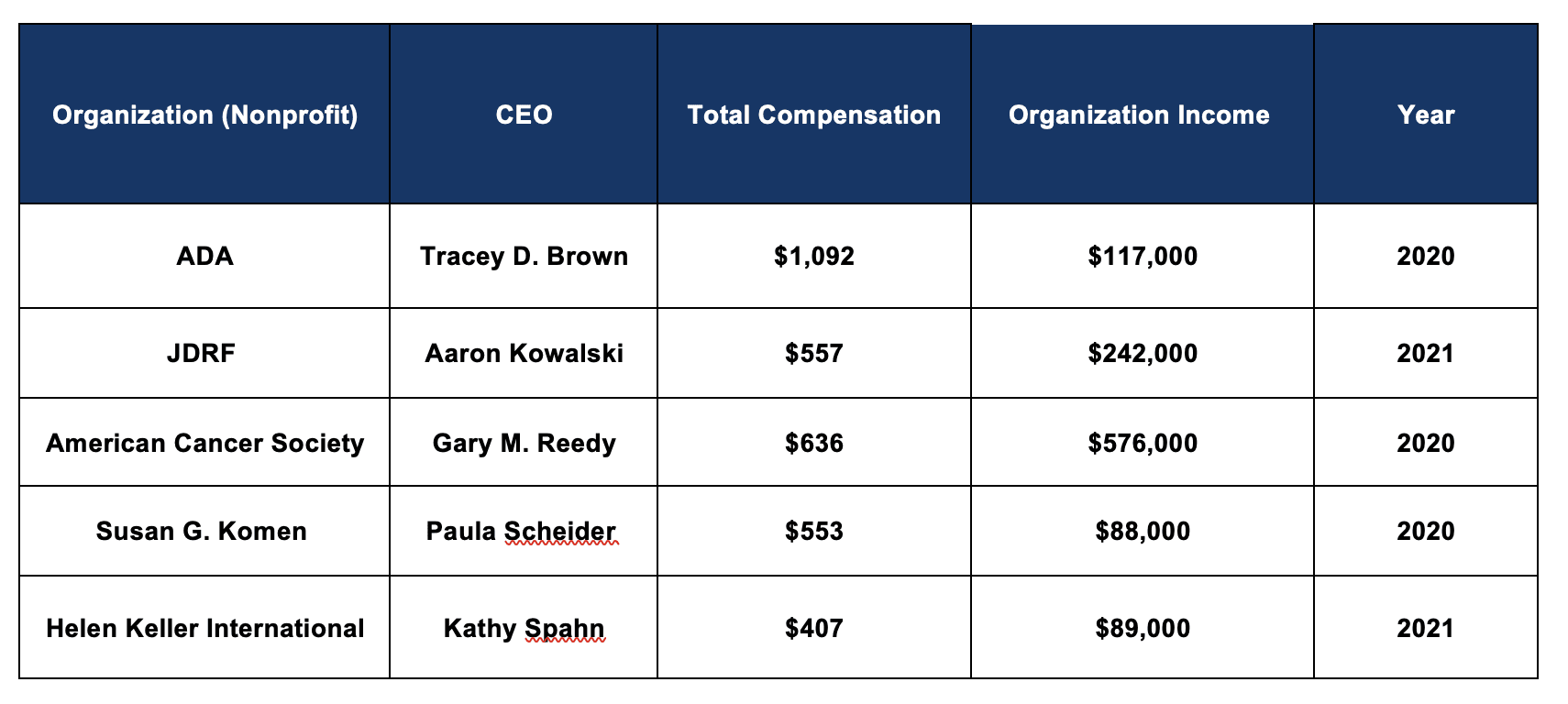
Diabetes nonprofit executives are among the highest paid in their field, topped by ADA CEO Tracey Brown who made over $1 million in Fiscal Year (FY) 2020. These leaders are within the top 1% of earners in the United States and earn more than the highest level executives at similarly sized nonprofits.
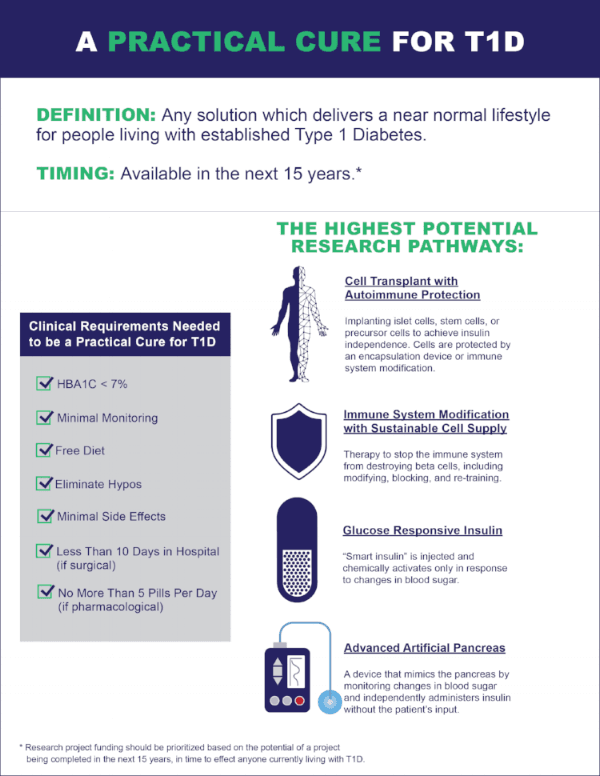
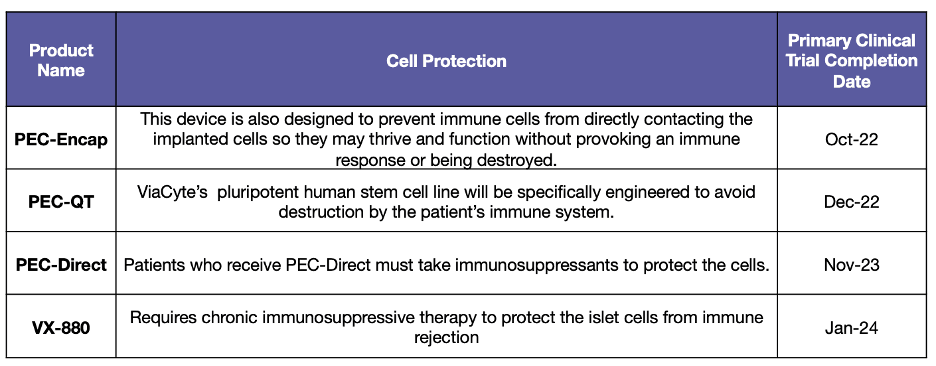
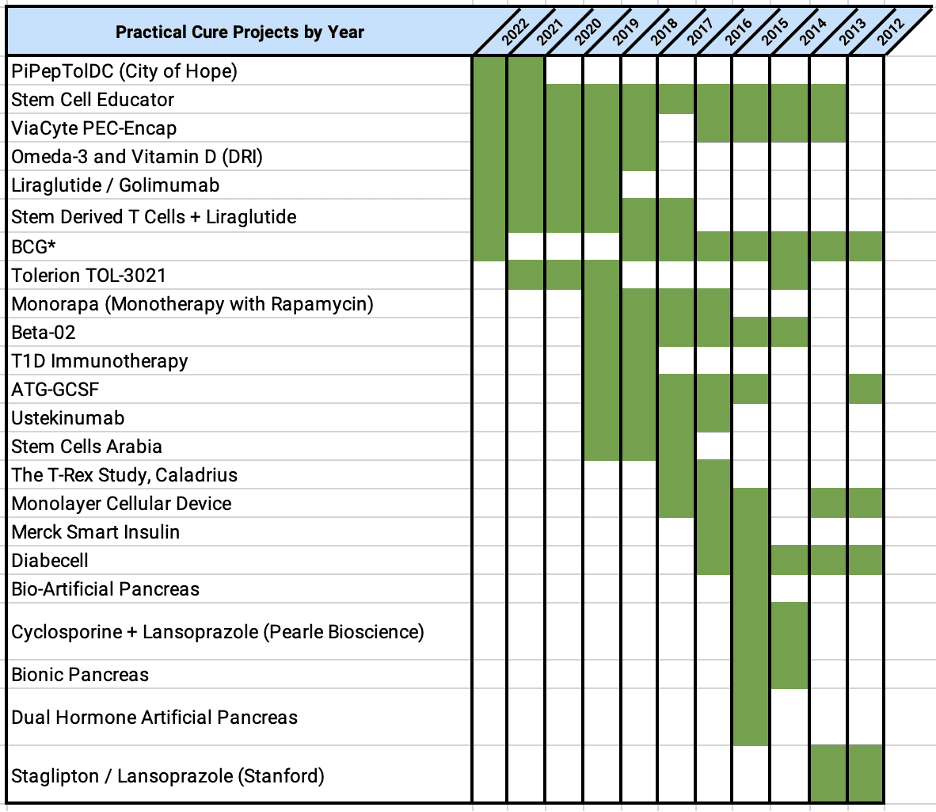
This report identifies the eight projects currently in human trials that have the potential to become a Practical Cure for T1D and to be available in the next 15 years.
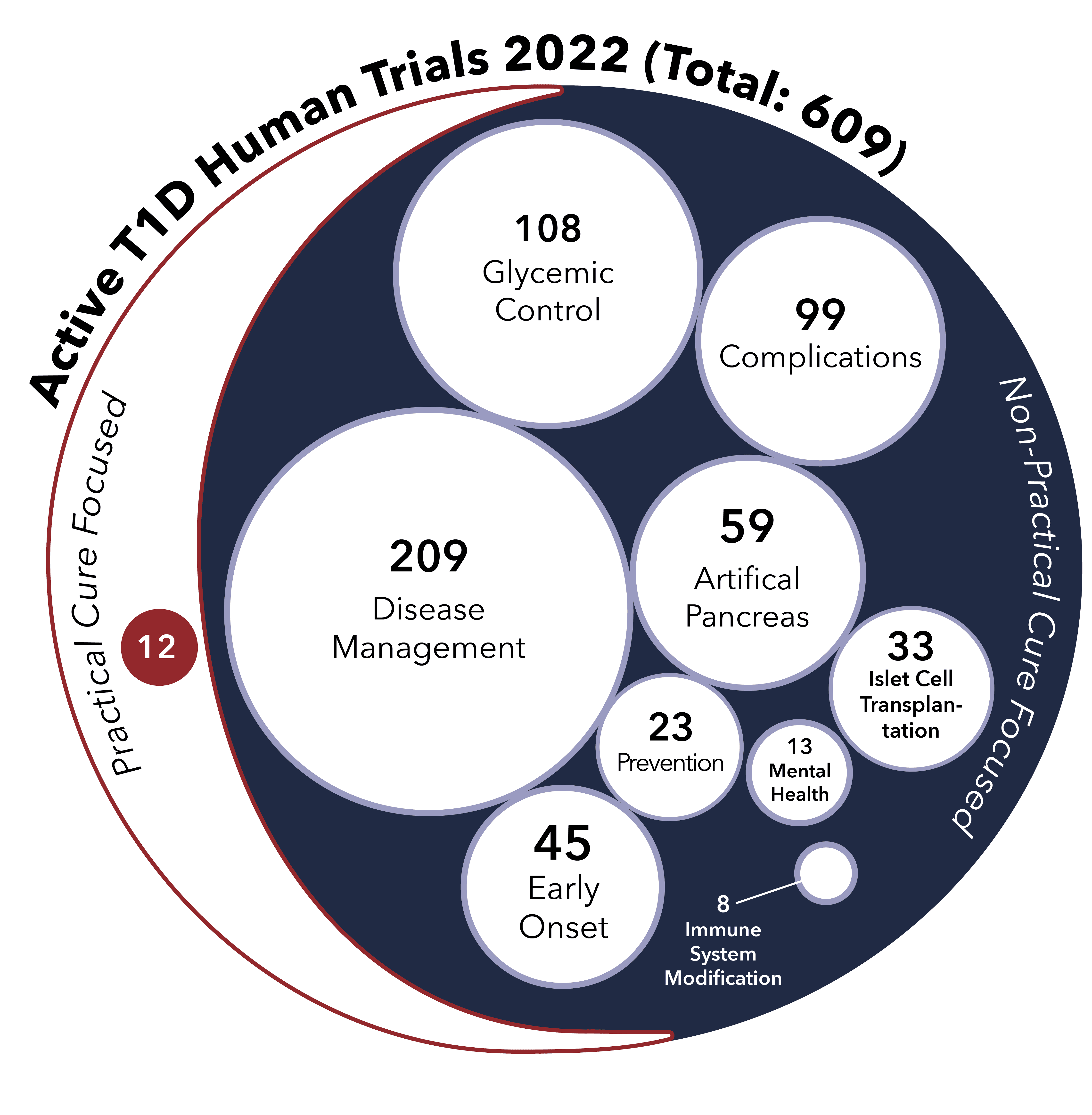
This report provides an overview of all the type 1 diabetes research projects currently active in human trials registered with the US FDA.
JDRF’s 2021 Fiscal Year endured the full force of COVID-19, stretching from July 2020 to June 2021. During this period, many organizations saw a contraction in revenue and, in response, reduced expenses substantially. JDRF cut expenses but, at the same time, surprisingly, experienced near record-high income.
This morning, Vertex Pharmaceuticals announced that it will acquire the private biotech startup ViaCyte for $320 million in cash. The deal will bring together two companies that have been competing to develop a Practical Cure for T1D.
Vertex Pharmaceuticals announced on Tuesday that the FDA will allow the company to continue its human trial of VX-880, the novel line of insulin-producing cells derived from stem cells.
Last weekend, The Human Trial, a must-see film for anyone interested in the effort to cure type 1 diabetes, opened in select cities across the United States.
This report will analyze the American Diabetes Association (ADA) Fiscal Year 2021 Audited Financial Statements, which cover a period from January 1, 2021 to December 31, 2021.
The American Diabetes Association (ADA) hosted its 82nd annual Scientific Sessions last weekend. It’s one of the year’s most important events for T1D research, bringing together many of the top researchers from around the world to publish and discuss their latest findings.
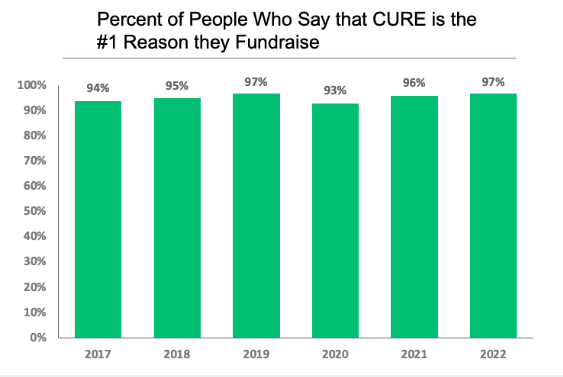
Each year, the individuals within the T1D community contribute nearly half a billion dollars to major T1D research centers and to diabetes non-profit organizations. What do these donors want when they make their generous donations? For over a decade, the JDCA has conducted annual surveys to answer that question.
Last week, the University of Alberta research team that published the breakthrough "Edmonton Protocol" in the year 2000 shared 20-years of data from its trial of islet cell transplantation therapy in patients with type 1 diabetes.
Last week, the American Diabetes Association (ADA) announced Charles D. “Chuck” Henderson as its new Chief Executive Officer (CEO). Henderson, a former Texas A&M basketball player, joined the ADA as Chief Development Officer in 2020 and has served as its interim CEO for the last six months.
Yesterday JDRF released financial statements from its most recent fiscal year, FY 2021 (which started July 1, 2020 and ended on June 30, 2021). The financial information is extraordinary because it reflects a full year hampered by the COVID-19 pandemic.
Yesterday, Vertex Pharmaceuticals shared updates from its active human trial of a stem-cell solution for T1D. This trial was all over the news last fall when the company published early results from a single patient who demonstrated a dramatic reduction in insulin use 90 days after receiving the treatment.
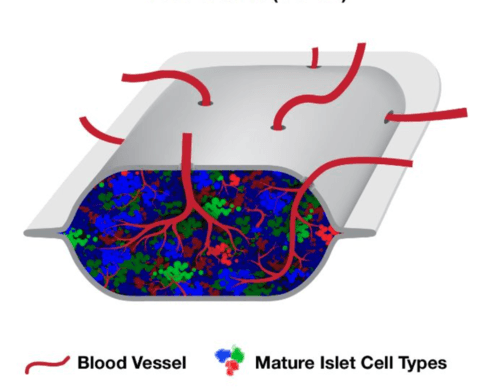
The type 1 diabetes (T1D) Practical Cure pathway which has received the most media coverage lately has been the development of stem cell therapies for T1D. These treatments offer hope that insulin-producing cells derived from stem cells could someday replace the need to manage T1D with insulin.
Last week, Doug Melton, the well-known Harvard professor who pioneered stem-cell therapies for T1D, announced that he will be leaving his university perch to join Vertex Pharmaceuticals. Melton will take a full-time position in the cell and gene therapy department with the title “Distinguished Vertex Fellow.”
This month, Denise Faustman’s lab at Massachusetts General Hospital (“MGH”) started a new chapter of its decades-long investigation of the Bacillus Calmette–Guerin (“BCG”) vaccine in type 1 diabetes (“T1D”) patients.
The American Diabetes Association ('ADA') is looking for a new CEO; no matter who is chosen, they will face a series of historic decisions.
This January, we published the 10th annual State of the Cure for Type 1 Diabetes. In recognition of this decennial anniversary, we take a moment to reflect on the progress of the past ten years toward achieving a Practical Cure for type 1 diabetes. During this period, T1D donors gave roughly $5 billion to T1D non-profit organizations and research centers. This report will address the return on that investment.
One of the most talked-about companies pursuing a T1D Practical Cure is ViaCyte, the San Diego company that hopes to use stem cells to provide a functional cure for T1D.
This morning, ViaCyte and CRISPR Therapeutics announced that the first patient has been dosed in their human trial testing a gene-edited, stem cell-derived product that evades detection by the immune system and produces insulin in people with T1D.
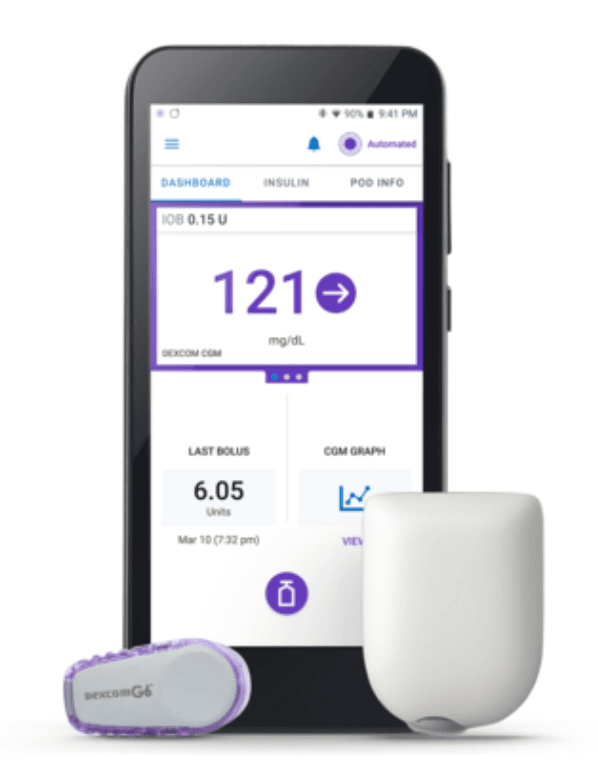
Last Friday, Insulet Corporation announced it received clearance from the US Food & Drug Association (FDA) to market its Omnipod 5 Automated Insulin Delivery (AID) system to people ages 6 and up with T1D.
This is the tenth annual edition of this report. It summarizes progress made during 2021 toward a Practical Cure for type 1 diabetes.