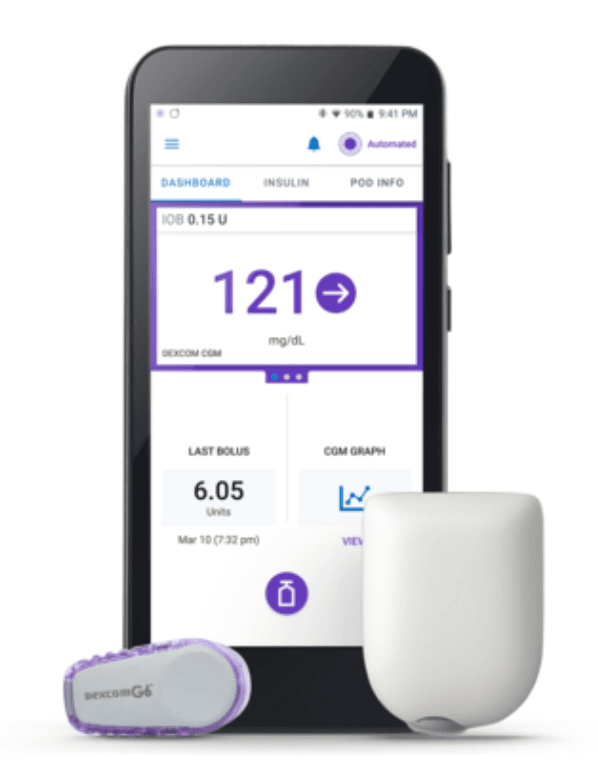
Last Friday, Insulet Corporation announced it received clearance from the US Food & Drug Association (FDA) to market its Omnipod 5 Automated Insulin Delivery (AID) system to people ages 6 and up with T1D.
When it enters the market later this year, the Omnipod 5 will be the first tubeless system approved by the FDA that can deliver insulin based on readings from a continuous glucose monitor (CGM). Unlike the AID systems currently on the market, insulin in the Omnipod 5 is stored in a reservoir that is attached directly to the body.
For fans of tubeless insulin pumps, which may offer increased freedom of movement for many people when compared to pumps with tubes, this is a big breakthrough. This product will pair all the benefits of a tubeless pump with a semi-automated system that can be monitored on your phone.
However, the moniker “artificial pancreas,” which is often ascribed to devices like this one, is likely to create a bigger splash than the story warrants. While some may find that the Omnipod 5 helps keep their blood glucose in range, the device is far from a Practical Cure. The ultimate burden of managing the disease – with this and all insulin delivery systems approved to date – still rests on the shoulders of the person controlling the device.
Key Summary Points:
- The Omnipod 5 approval is a breakthrough for people who are fans of tubeless pumps (though people have been unofficially 'looping' their Omnipods with Dexcom G6's for a number of years).
- It does not replace the function of the pancreas.
- It is not a Practical Cure, nor is it a full closed-loop artificial pancreas.
- We expect this technology will be further iterated to be smaller and offer more freedom of movement in the years ahead.
What is the Technology?
- The Omnipod 5 System consists of the tubeless Pod, the Dexcom G6 continuous glucose monitor (CGM), and the Omnipod 5 mobile app with its integrated SmartBolus Calculator.
- The user has the option to download this app onto a compatible personal smartphone or to use the Omnipod 5 Controller.
- Every five minutes, the software receives a Dexcom CGM value and trend and predicts where glucose will be 60 minutes into the future. The system then increases, decreases, or pauses insulin delivery using the user’s desired and customized glucose target, helping to protect against highs and lows.
- While the system can automatically "treat to target" glucose, the user still needs to manually input carbohydrates or exercise.
- It is worth noting that people with T1D have been “looping” their continuous glucose monitors with insulin pumps for years. The latest iteration of AID pumps is only the first wave of FDA-approved products.
How Close is this to a Practical Cure for T1D?
This device is not a Practical Cure. Much of the media coverage suggests that a switch to the Omnipod 5 will lift the burden of managing T1D. However, most will, no doubt, be disappointed by this promise. That said, this device is the first tubeless AID pump approved by the FDA. We expect that this will pave the way for future developments in AID technology. If Moore's Law holds, we look forward to it becoming half its current size and twice as powerful in the next several years. And we expect that this approval will set the stage for other competitors.
But to be a Practical Cure it needs to be small enough, effective enough, and trustworthy enough to set it and forget it. Our surveys indicate that, for most people, the Omnipod 5 will not meet this bar. The current system still requires that the user wear two clunky devices – an insulin “patch” which is only a little smaller than an Apple AirPods case, and a continuous glucose monitor. Furthermore, the person managing the disease is still the “brains” of the operation; they still carry the burden of alerting the system when it is time to eat, exercise, etc.
In our view, the shortcomings with the latest generation of mechanical AID systems point to opportunities to improve. We hope that soon there will be FDA clearances of devices that are small enough and smart enough to deliver a Practical Cure for T1D.
