
FDA approval of a closed-loop artificial pancreas has generated significant press and media coverage during the past 24 hours. This is the first closed-loop AP approved for broad use in the United States and is a landmark event, paving the way for future devices advanced enough to be a Practical Cure.
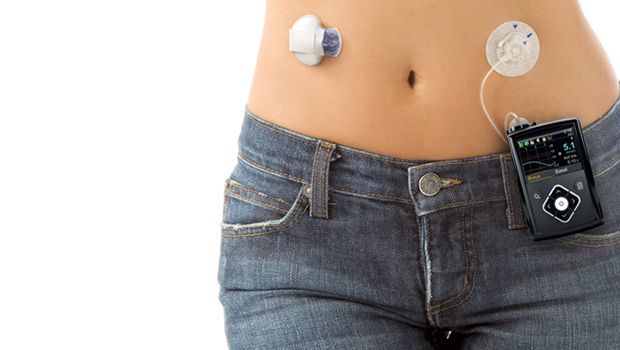
Medtronic’s MiniMed 670G hybrid closed-loop artificial pancreas system received approval from the U.S. Food and Drug Administration on Wednesday. The device combines the ability to automatically monitor glucose and administers a continuous rate of insulin. It includes a CGM sensor attached to the body to measure glucose, an insulin pump also strapped to the body, and an infusion patch attached to the pump with a small catheter.
HIGHLIGHT POINTS:
- Expected to be available in the market during the Spring of 2017.
- It is approved for use only in patients aged 14+.
- Clinical trials are currently underway for patients under 14.
- Insulin only. Does not dispense glucagon (as the Bionic Pancreas hopes to do).
- Utilizes an algorithm that learns and adapts to each person's unique needs.
- Still requires manual carbohydrate entry at meal times.
- Also requires the patient to accept bolus correction recommendations.
- Automatically shuts off during hypo events.
A PRACTICAL CURE?
While this is a great step in the right direction, and one that will improve the lives of many people, it is not yet a Practical Cure. However, this should point the way to the second and third generations of AP devices that will reach the mark: Small enough in size to forget they are wearing it and analytically powerful enough to be fully automated.
RELATED LINKS:
FDA Press Release: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm
Medtronic Press Release: http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=2206594
