This report focuses on ViaCyte’s islet cell replacement therapies, identified as a Practical Cure in the 2018 State of the Cure for T1D, and features an interview with ViaCyte president and CEO Paul Laikind. ViaCyte Inc. is a for-profit company located in California.
Key Highlights
- ViaCyte is actively testing a potential practical cure for T1D in human trials. They address cell supply through their own line of stem cells (designated stem cell line “CyT49”) and cell protection through their own encapsulation device.
- The stem cell line provides a potential solution to current islet cell transplant approaches which are restricted by extremely limited availability of pancreases from cadavers.
- The company is testing two different product candidates:
- PEC-Encap: The device used in this product candidate is designed to protect the cells from the immune system and thus does not require immune suppression. PEC-Encap is considered a full Practical Cure with potential application to all type 1 diabetes patients.
- PEC Direct: The device used in this product candidate allows direct vascularization of the cells and requires prolonged immune suppression. Because of the associated risks of immunosuppression, the product candidate is limited to only high risk T1D patients and is not considered a Practical Cure.
- PEC-Direct is being tested in a human trial in the United States and Canada. The company also announced the start of a European Trial in January of 2019.
- The PEC-Encap trial was “paused” in 2016 due to low levels of engraftment. The company has been working with W.L. Gore to improve its encapsulation device material and hopes this will solve earlier challenges. Clinical development is expected to resume in the Fall of 2019, according to Laikind.
- Laikind also discussed ViaCyte’s recently announced research collaboration with CRISPR Therapeutics, a biopharmaceutical company, which will focus on engineering a stem cell line that is resistant to the T1D autoimmune response. This collaboration is still in very early stage testing.
Research Background:
ViaCyte has developed their own line of pancreatic progenitor cells, derived from an embryonic stem cell line, that once implanted are expected to develop into insulin-producing islets. Because the stem cells are capable of self-renewing and can be directed to form insulin producing cells, the hypothesis is that this will resolve the issue of cell supply. Assuming successful delivery and engraftment, this could potentially eliminate the need for insulin completely. As noted in the opening bullets, ViaCyte is also developing two devices to deliver and house the cells for the PEC-Encap and PEC-Direct product candidates.
PEC-Encap
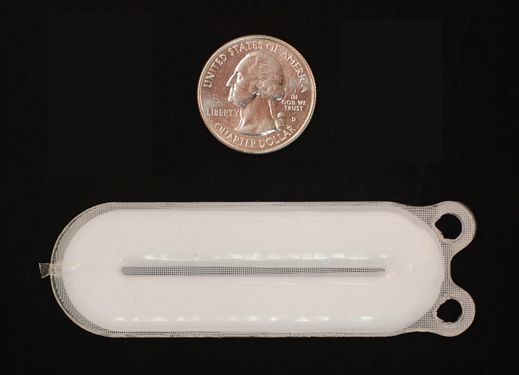
The PEC-Encap approach utilizes an encapsulation device called the Encaptra Cell Delivery System which houses the cells in a thin, flexible device about half the size of a credit card and is implanted just under the skin. The encapsulation device has two main functions: first, the device is designed to protect the cells from both the normal immune response the body mounts against “foreign” implanted cells as well as the T1D autoimmune attack using a protective membrane, or outer device layer. Second, given that the membrane doesn’t allow cells, including blood vessels to pass through, the device is designed to support blood vessel formation along the device surface thus supplying the cells with oxygen and nutrients. This enables the delivery of insulin and other regulatory hormones from the cells to the rest of the body.
ViaCyte began testing PEC-Encap in 2014 in adults with established T1D in a phase I/II trial at centers in the U.S. and Canada. All patients in the PEC-Encap trial have established T1D (at least three years since diagnosis with no detectable beta cell function). As noted in the opening bullets, in early 2016 the trial was “paused due to variability in the engraftment process.” Subsequently, ViaCyte announced a collaborative research agreement with W. L. Gore, a materials science company, in March 2017 to pursue alternative materials and designs with the goal of improving engraftment and now plans to resume enrollment in the third quarter of 2019. ViaCyte also initiated a second phase of the PEC-Encap trial in 2016 to evaluate the long-term safety of PEC-Encap.
ViaCyte reported two-year data on PEC-Encap in 2018 at the ADA conference which showed the product was safe but had limited cell survival rates. In a press release, Laikind quoted, “When engraftment did occur, viable mature insulin-expressing endocrine islet cells were formed. In some cases, insulin-expressing cells persisted for up to two years after implantation, the longest time point investigated in the study.”
ViaCyte’s Encaptra Cell Delivery System
PEC-Direct
PEC-Direct is designed to allow blood vessels to directly interact with the cells. Because of the open nature of the device, treatment with PEC-Direct will necessitate the use of traditional immune suppression drugs to protect the cells from the autoimmune attack. Laikind described the immune regimen as largely the same as the treatment required for standard islet cell transplantation. This approach has been identified by ViaCyte as particularly applicable for high-risk T1D patients with a high incidence of severe hypoglycemic episodes associated with impaired awareness of hypoglycemia (IAH) and patients with extreme glycemic lability.
ViaCyte began testing its PEC-Direct product in a phase I/II trial in 2017. The first phase of the trial, which tested safety, was completed last year. A second phase of the trial was initiated in 2017. This trial remains ongoing and has a goal of testing both safety and efficacy. In January 2019, ViaCyte announced that it has also started a PEC-Direct trial in Europe in collaboration with the Center for Beta Cell Therapy in Diabetes, a translational medicine diabetes consortium.
