
September 14, 2023
Summary Insights:
- 627 T1D trials registered in the United States as of July 2023.
- 13 of those trials have the potential to become a Practical/Functional Cure.
- 2 new Practical Cure projects.
- 4 active trials led by Vertex Pharmaceuticals (including those still listed under the company 'ViaCyte' that Vertex acquired last year).
This report identifies the 10 projects currently in human trials and registered in the United States that have the potential to become a Practical Cure for T1D within the next 15 years. These 10 projects are being tested in 13 different trials. Each project tests a specific scientific thesis that could one day become a T1D Practical Cure. A single project may have multiple trials running concurrently that vary slightly (testing different age groups, higher/lower doses, etc.).
Projects included in this list are testing both a sustainable cell supply and a cell protection solution. While there are many additional projects testing only one part of the equation, this list includes only those that contain both components.
This list is not an endorsement or a validation of the effectiveness of the research. Instead, its purpose is to show and record the projects in human testing which, if proven effective, have the potential to be a Practical Cure.
Note: JDCA’s 2023 “Annual Review of T1D Clinical Trials” miscategorized ViaCyte’s VCTX-211 as islet cell transplantation. It is, in fact, a Practical Cure project. While this resulted in an insignificant percentage change across trial categories, this is significant for Practical Cure projects, of which there are so few. An updated version of the 2023 report can be found here.
What is a Practical Cure?
A Practical Cure is any solution that minimizes the disruptive aspects of T1D and delivers the return to a quality of life similar to pre-diagnosis without relying on immunosuppression, constant monitoring, or risking adverse side effects. A Practical Cure (sometimes referred to as a Functional Cure) differs from an idealized, perfect cure in that it does not result in the reversal or complete elimination of the disease.
This definition of a Practical Cure was established by a consensus of individuals currently living with T1D. It expresses their vision of a life where most of the daily burden of T1D is alleviated. One great benefit of the Practical Cure—in contrast to a perfect cure—is that it has the real potential to be available in our lifetime.
Highlights: New Arrivals, Ongoing Projects, and Removed Trials
There have been several changes in Practical Cure projects since the previous review conducted in November 2022, in our annual publication, State of the Cure (SOTC). At that time, there were 8 Practical Cure projects in 12 human trials. Today, there are 10 projects in 13 human trials. During the past year, all 8 Practical Cure projects from 2022 continue onward, 2 new projects have begun, and 2 trials were removed.
New Practical Cure Projects
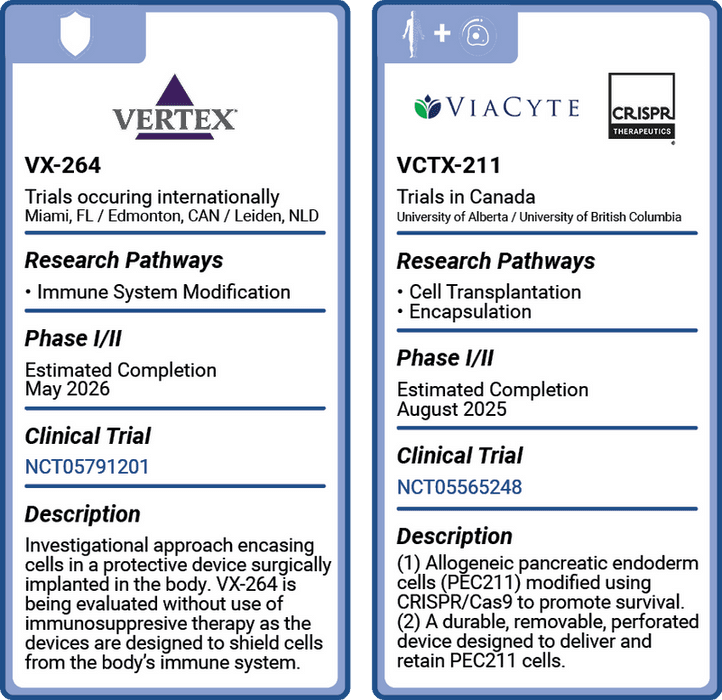
VX-264: NCT05791201; VCTX-211: NCT05565248.
Vertex: VX-264
VX-264 brings an encapsulation cell protection solution to its stem-cell line of insulin-producing cells. VX-264 includes an encapsulation device designed specifically to protect Vertex’s stem cell-derived, fully differentiated, insulin-producing islet cells from autoimmune attack, negating the need for immunosuppressants. These are the same islet cells tested in Vertex’s VX-880 that presented positive clinical data earlier this year. VX-880 acts as a precursor to VX-264 and is within itself a powerful potential Practical Cure component.
This is being tested in one Phase II trial occurring in several global locations: Florida, Canada, and the Netherlands. The trial began in May of this year and has an estimated completion date of August 2025.
'ViaCyte' & CRISPR: VCTX-211*
VCTX-211 combines ViaCyte’s stem-cell line of insulin-producing cells with CRISPR Cas9 gene editing to resist the autoimmune attack. It is testing two components: (1) allogeneic pancreatic endoderm cells (PEC211) that have been genetically modified with CRISPR Cas9 gene editing technology to evade the immune system and ensure cell fitness; (2) a durable encapsulation device designed to deliver, house, and protect PEC211 cells. This is different from ViaCyte’s other Practical Cure project that tests PEC cells without CRISPR Cas9 gene editing.
One clinical trial is taking place at the University of Alberta and the University of British Columbia in Canada to test safety, tolerability, and efficacy. This Phase I/II study began in January of this year and has an estimated completion date of August 2025.
*In July 2022, ViaCyte was acquired by Vertex. All projects/trials listed as ‘ViaCyte’ are now produced by Vertex. However, at the time of this publication, trial names and projects started by ViaCyte remain listed in clinicaltrials.gov as such.
Ongoing Practical Cure Projects

Top Row: Omega 3 and Vitamin D: NCT03406897; MSC-toIDL: NCT05207995; OPERA Study: NCT05413005; T Cells + Liraglutide: NCT03011021.
Bottom Row: PIpepToIDC: NCT04590872; Pancreatic Endoderm Cells: NCT02939118, NCT03163511; Repeat BCG Vaccinations for the Treatment of T1D: NCT05180591, NCT02081326; Stem Cell Educator: NCT04011020, NCT02624804.
The infographic above shows the 8 Practical Cure projects that continued onward into 2023. Each project active in 2022 remains so today. However, some individual trials within the projects have shifted.
The Practical Cure project, Stem Cell Educator, added one new trial in December of last year. In addition to this, the Faustman Lab has gained approval for an expanded study arm for repeat dosing of BCG in adult T1D.
Removed Trials
During the same period, two trials were removed. Both trials are a part of Vertex/ViaCyte’s Pancreatic Endoderm Cells program. They are as follows:
VC01-103
NCT04678557
The trial tested safety, tolerability, and efficacy for 1 year. The results mark the overall status of this trial as terminated for “insufficient functional product engraftment.” This information is flagged in the clinical trials database as the official quality control review has not concluded, therefore it is technically marked as “Active, Not Recruiting” until the review is complete.
VCTX210a
NCT05210530
A Phase I trial evaluating safety and tolerability has been removed and marked “completed” in the clinical trials database. At this time, there are no results posted.
Reference: Practical Cure Projects Actively Recruiting
- VCTX-211
- VX-264
- Stem Cell Educator (specifically trial #NCT04011020)
- Faustman Lab (specifically trial #NCT05180591)
- Omega-3 & Vitamin D in High-Dose
- OPERA Study
- Umbilical T Cells + Liraglutide
- PIpepToIDC
