
At a Glance
- 2% of all current T1D clinical trials are potential Practical Cures (as of June 2024).
- 10 trials (8 projects) have the potential to become a Practical Cure.
- 1 new Practical Cure project.
- 4 trials (3 projects) from last year have been completed and removed.
- The total number of Practical Cure versus all T1D trials has never risen above 3% in any given year. This must change.
August 8, 2024
This report provides a comprehensive overview of the Practical Cure projects currently active in human trials and registered with the US FDA, concluding with a perspective on what has changed in the last twelve years. It is important to note that one Practical Cure project can take place in several human trials by testing the same therapy with alternative criteria (e.g., different age groups, higher/lower doses, etc.). There are eight registered research projects taking place in ten human trials. Since JDCA’s clinical trial categorization overview last month, one Practical Cure project has been completed and thus removed from the list.
To qualify as a Practical Cure project, all trials must test a way to increase insulin-producing beta cells in the body (via cell therapies, transplantation, regeneration, etc.) and provide these cells protection from the autoimmune attack without lifelong immunosuppression. These trials, if successful, could provide a solution for everyone in the T1D community, regardless of health, age, or time since diagnosis.
This list is not an endorsement or validation of the effectiveness of the research projects. The purpose is to record and present the projects currently in human trials that, if proven effective, could become a Practical Cure for T1D. A summary of research pathways to a Practical Cure is in Appendix A.
Practical Cure Highlights: Continued Research, A New Arrival, and Removed Projects
There have been a few changes in Practical Cure projects since our 2023 review, updated in the annual State of the Cure for Type 1 Diabetes. At that time, there were ten projects taking place in thirteen human trials. Since then, one new project has begun, seven projects continue into this year, and three projects were removed.
Continued Projects
Seven of the ten active Practical Cure projects continue from last year, all detailed in past reports (see summary figure 1). Most of the projects are simply ongoing, without material news to report, but two have notable developments.
Of note is that only a few stem cell-derived beta cell solutions are active in the list. While this pathway has received significant media attention over the past several years and many researchers are testing solutions pre-clinical, only a handful have made it to human trials. Vertex, who acquired ViaCyte in 2022, continues making headway, though we welcome increased competition and hope to see more human trials along this pathway added to this list.
Figure 1: Summary of Returning Practical Cure Projects

Two projects have had notable developments since our last report:
Throne Biotechnologies: Stem Cell Educator
The project received Regenerative Medicine Advanced Therapy designation from the FDA in April of this year. This designation should fast-track Stem Cell Educator based on new/intermediate endpoints.
Viacyte: VCXT-211
Previously a collaboration between CRISPR Therapeutics and Vertex Pharmaceuticals, the trial is now wholly owned by the former after the partnership ended in January of this year. The clinical trial database continues using the name ‘VCTX-211,’ though the trial is referred to as ‘CTX-211’ in all CRISPR media. Other collaborations between the two organizations—including the research and development of hypoimmune cells for T1D—were not affected.
New Project: Sana Biotech & Uppsala University, Hypoimmune Islets
Figure 2: New Practical Cure Project

The sole addition to this year’s Practical Cure list aims to cure T1D via Gene-Edited Cells and Cell Transplantation Pathways.
This early phase I trial is spearheaded by Prof. Per-Ola Carlsson of Uppsala University Hospital in collaboration with Sana Biotechnologies. Insulin-producing donor cells are genetically modified using Sana’s technology to become hypoimmune—innately invisible to the immune system—before being implanted into the forearm muscle of study participants. The hope is that these modified cells will evade detection by the immune system and negate the need for immunosuppressants.
The study began in February of this year and is expected to conclude in May 2025. This is a small safety trial, with only two participants enrolled, taking place at Uppsala University Hospital in Sweden.
Removed Projects
Within the last year, three projects taking place in four trials were completed and accordingly have been removed from the Practical Cure list.
ViaCyte: VC-01 and VC-02
Both trials, part of the Pancreatic Endoderm Cell project line, were marked “Completed” on the clinical trial database after the studies' end dates were met. VC-02 was an interventional phase I/II trial evaluating the efficacy of transplanted PEC-Direct cells (embryonic stem cell-derived beta cells) in an encapsulation device. VC-01 was an observational study evaluating the long-term safety of subjects previously implanted with PEC-Direct.
Results have not yet been posted. Following JDCA inquiry, the company did not offer trial results or comment on the next steps. Usually, a lack of results is not a good sign, as most want to share strong results quickly. It is unclear if the VC-01 PEC-Direct line will move into phase III clinical trials and return to the Practical Cure list next year.
Diabetes Research Institute: POSEIDON
Phase I/II trial tested the combination therapy of Omega-3 Fatty Acids and Vitamin D. The drug was given orally to promote sustained immune regulation, reduce inflammation, and support residual beta cell mass. The study, whose duration was estimated to end December 2026, was marked “Completed” last month. We do not yet know if the trial will progress to phase II/III.
National Academy of Sciences of Belarus: MSC-toIDC
Phase I/II trial tested the safety of a combination injection of dendritic cells, adult stem cells, and Vitamin D3 so the immune system will ignore beta cells and encourage cell regeneration. After inquiry, JDCA was informed the trial was completed with results that showed some increase in C-peptide. However, after further questioning, it was revealed participants required a C-peptide level at recruitment which indicated the trial was for new-onset patients rather than fully established.
Close Call: Trial Placed on FDA Hold
Biomea Fusion’s COVALENT-112 trial, on the Immune System Modification and Cell Regeneration pathways, was removed from the Practical Cure and clinical trial categorization list after the study was placed on hold by the FDA due to concerns of liver toxicity. The trial, with “Suspended” status on the clinical trial database, does not qualify as a Practical Cure since the trial is not active. If the hold is lifted and the trial is deemed safe, it will be added to the Practical Cure list.
Discussion on Historical Projects
Reference Appendix B for all Practical Cure project additions and removals since JDCA began tracking this information (2012).
How Have Practical Cure Projects Changed?
2024 marks the thirteenth year the JDCA has been tracking Practical Cure projects. In that time, we reported twenty-eight different projects in many more clinical trials. Of these, three projects have been removed from the list only to reappear later (BCG, Tolerion TOL-3021, ATG-GCSF). Every year, except 2019 and 2020, we’ve seen at least one new project.
The Immune System Modification pathway, both alone and alongside others, is the most frequent pathway taken in Practical Cure projects (61%) since 2012—five out of ten this year. Stem cell-derived beta cells, utilized in the Cell Transplantation pathway to replace the body’s lost insulin-producing cells, have gained traction in clinical research. The Advanced Artificial Pancreas and Glucose Responsive Insulin pathways are the least common themes, seen in only three projects spanning 2014-2016 (Merck Smart Insulin, Bionic Pancreas, Dual-Hormone Artificial Pancreas).
What Is Our Greatest Chance for a Practical Cure?
Every Practical Cure project, trial, and initiative is highly valued by the JDCA and the T1D community. Each one represents the hope of freedom from the burdens of the disease and the opportunity to regain a ‘normal’ quality of life—for everyone affected by T1D.
JDCA does not hold preference for one Practical Cure project over another or endorse the science. If a project is in human trials, it has the potential to become a fully realized Practical Cure within the next fifteen years. The results of each project—both intermittent and final—should speak for themselves.
Of 634 T1D clinical trials this year, only 10 trials (in 8 projects) qualify as a potential Practical Cure. This amounts to 2%. The percentage of Practical Cure trials out of all T1D trials has never risen above 3% since JDCA began tracking this information, despite the total number of T1D trials growing each year (91% increase from 2012 to 2024).
Our greatest chance for a Practical Cure is not held in any one project, but by having more fully-funded projects that can complement and compete with each other to expedite progress toward a cure. 2% of all trials is not enough. 3% of all trials is not enough. If the T1D community wants to see a Practical Cure within our lifetimes, the trend of stagnation cannot be allowed to continue. We must see a paradigm shift in research and funding that prioritizes a Practical Cure above all else.
T1D donors, the power is in your hands. Your donations fuel the fire in the diabetes research ecosystem. With our combined voices, we can influence diabetes nonprofits to focus funds on T1D cure research. By attaching a note or comment to your donation, large diabetes nonprofits are obligated to put your money where you intend it to go.
Reference: Practical Cure Projects Actively Recruiting
- Faustman Lab (specifically #NCT05180591)
- OPERA Study
- PIpepToIDC
- Stem Cell Educator (specifically #NCT04011020)
- Umbilical T Cell Plus Liraglutide
- VCTX-211
- VX-264
Appendix A: Practical Cure Pathway Definitions

Appendix B: Practical Cure Projects (2012-2024)
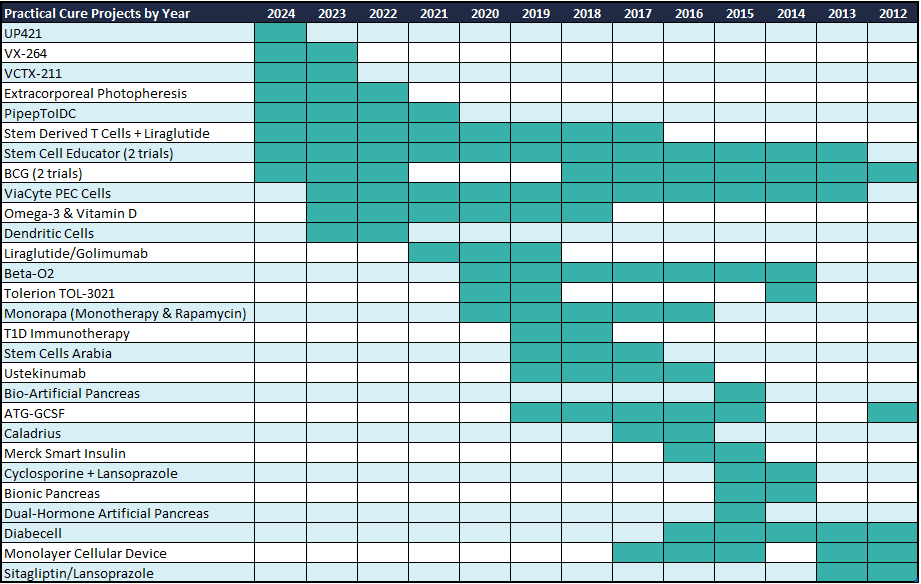
Note: Green rectangles indicate the lifetime of Practical Cure Projects. e.g., Stem Cell Educator began in 2013 and continues into 2024; BCG began in 2012, was removed in 2019, and returned in 2022.
